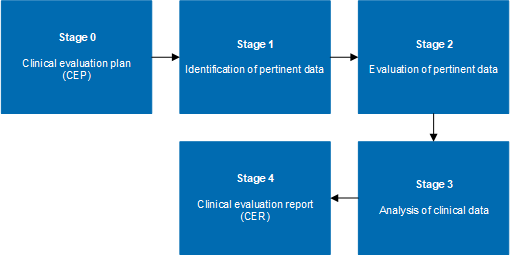
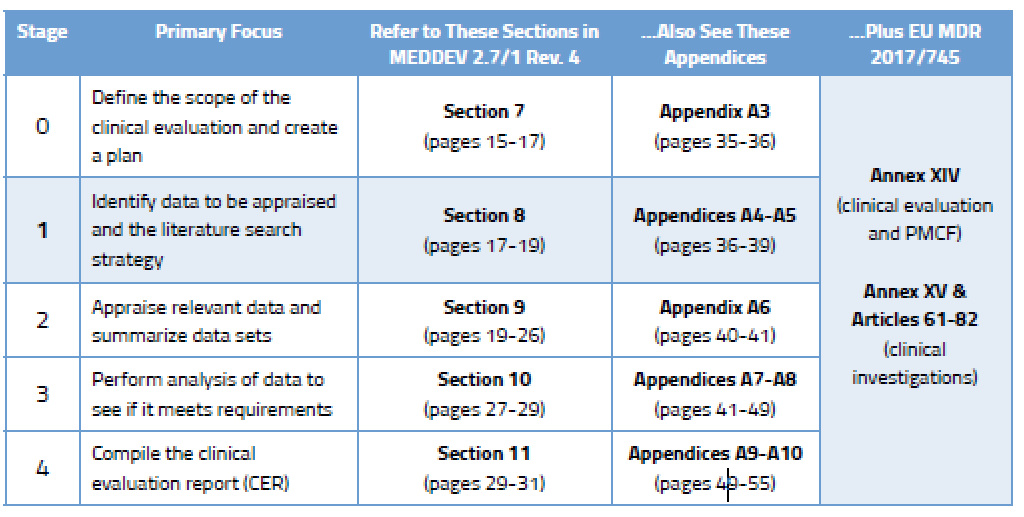
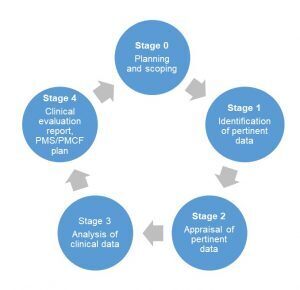
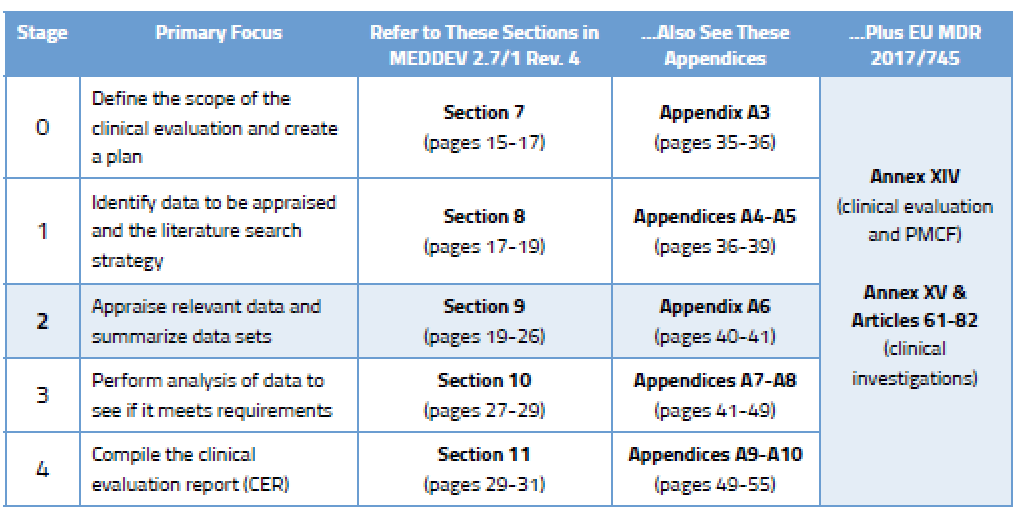
How to perform a clinical evaluation of medical devices – Part 1 – Overview and sample of activities – Medical Device Expert News

Does Your Organization's Post-Market Clinical Follow-Up (PMCF) Plan Adequately Reflect the Intensity Required in the Clinical Evaluation Report (CER) Under the Newest Medical Device Regulations? - Criterion Edge
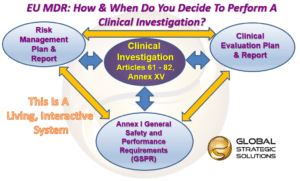
What Is The Difference Between Clinical Evaluation and Clinical Investigation? | Global Strategic Solutions

Guideline for following the latest Medical Device Regulation : case: wellbeing analytics company | Semantic Scholar

Importance of systematic literature search for clinical evaluation(ce) the strict adherence of medde by PepGra CRO - Issuu


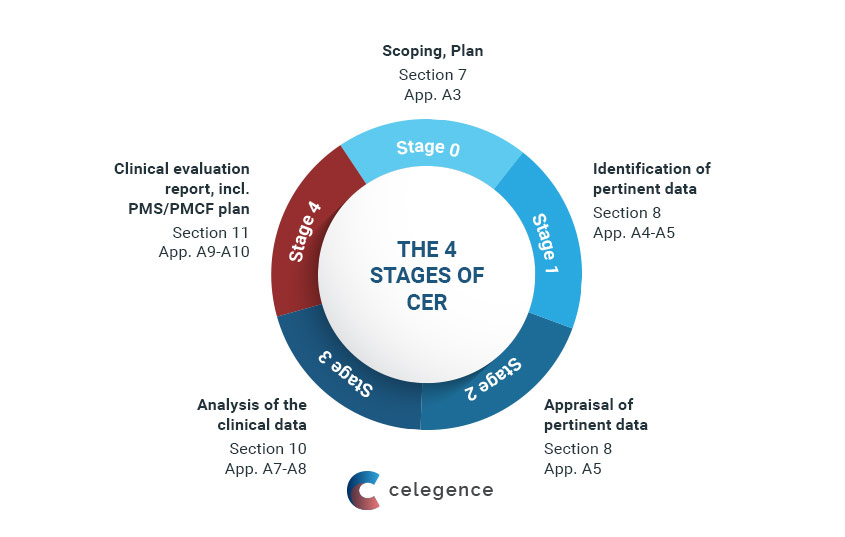




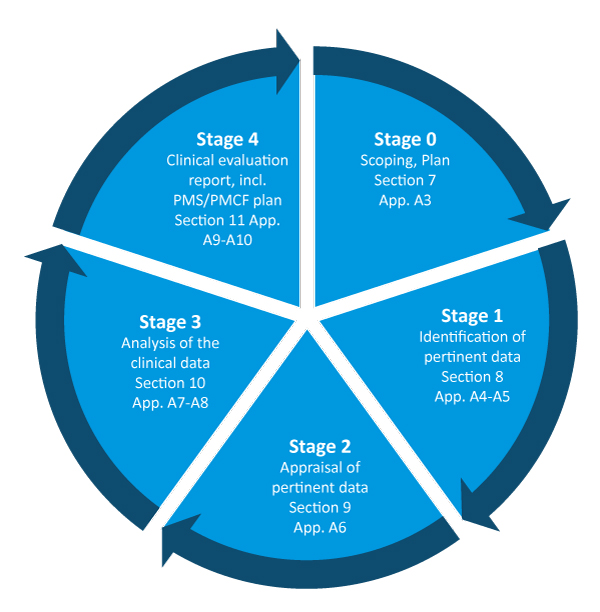



![Clinical Evaluation Plan [ISO 13485 templates] Clinical Evaluation Plan [ISO 13485 templates]](https://advisera.com/wp-content/uploads//sites/14/2021/08/19.1_Appendix_1_Clinical_Evaluation_Plan_Integrated_Preview_EN.png)