Adaptive designs in clinical trials: from scientific advice to marketing authorisation to the European Medicine Agency | Trials | Full Text
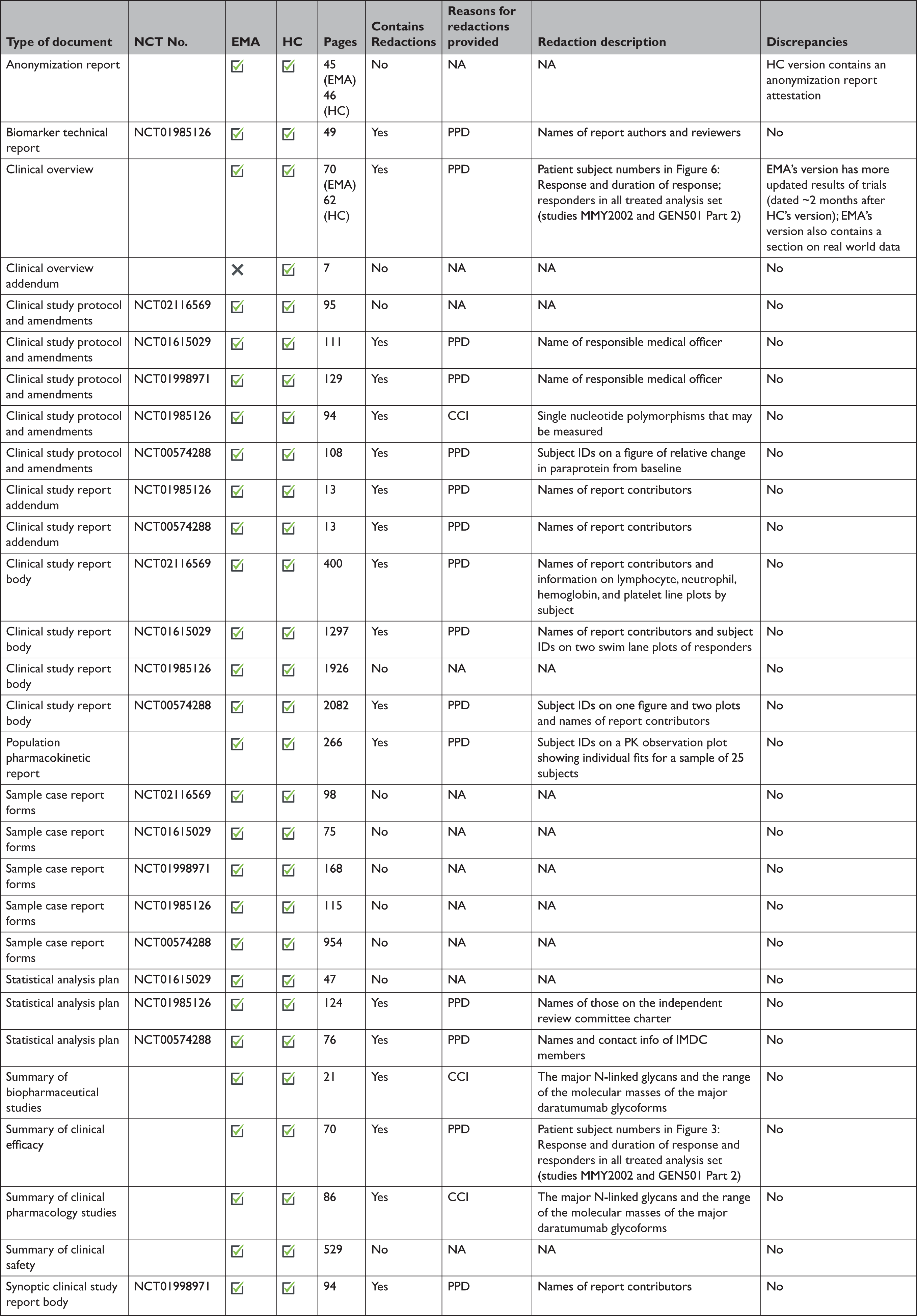
Transparency of Regulatory Data across the European Medicines Agency, Health Canada, and US Food and Drug Administration | Journal of Law, Medicine & Ethics | Cambridge Core

EMA report on geographic distribution of clinical trials supports need for revision of European clinical trial legislation

Risk of bias in industry-funded oseltamivir trials: comparison of core reports versus full clinical study reports | BMJ Open


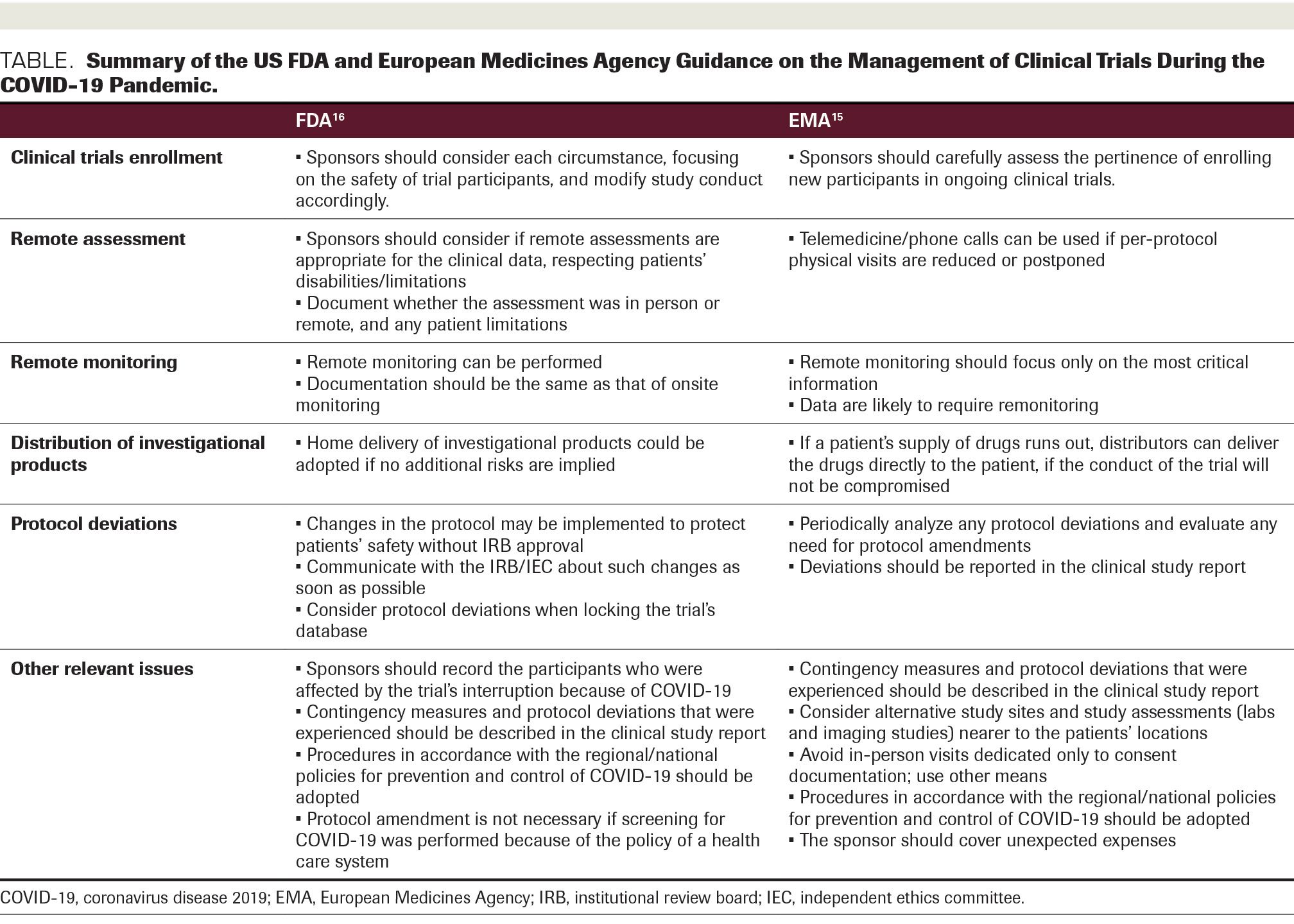


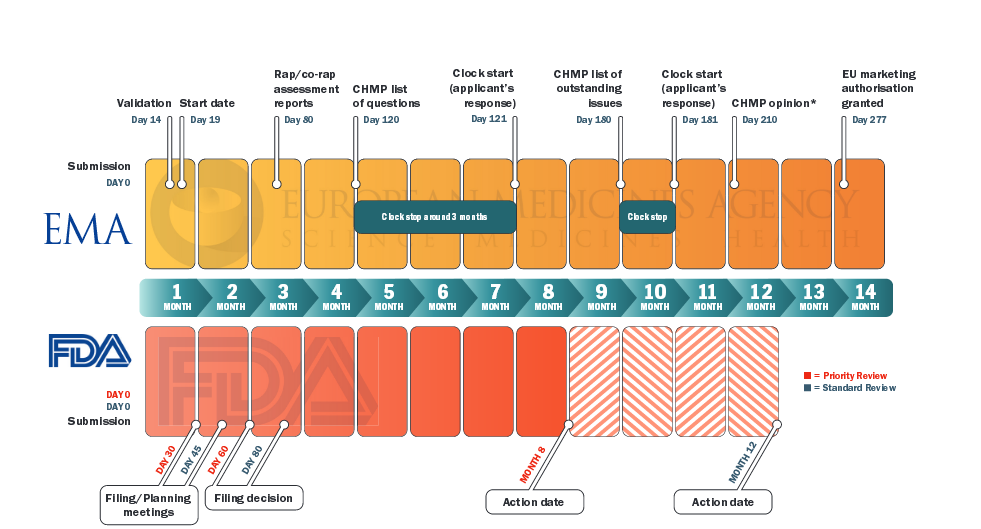


![PDF] Effective authoring of clinical study reports: A companion guide | Semantic Scholar PDF] Effective authoring of clinical study reports: A companion guide | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/2f8d38c9afbd7fce7a7635cf459a7989c82a753a/5-Table1-1.png)