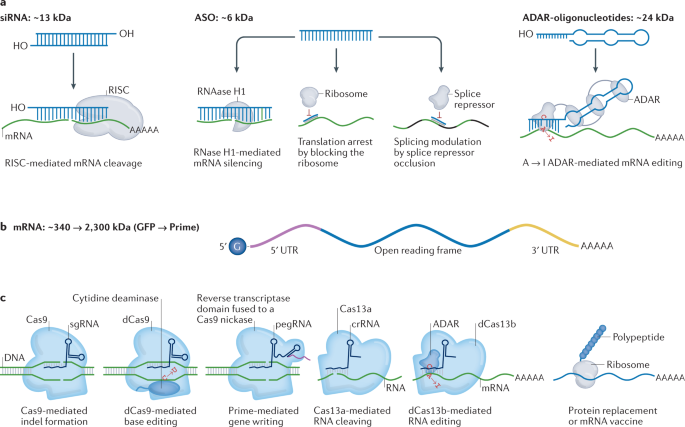
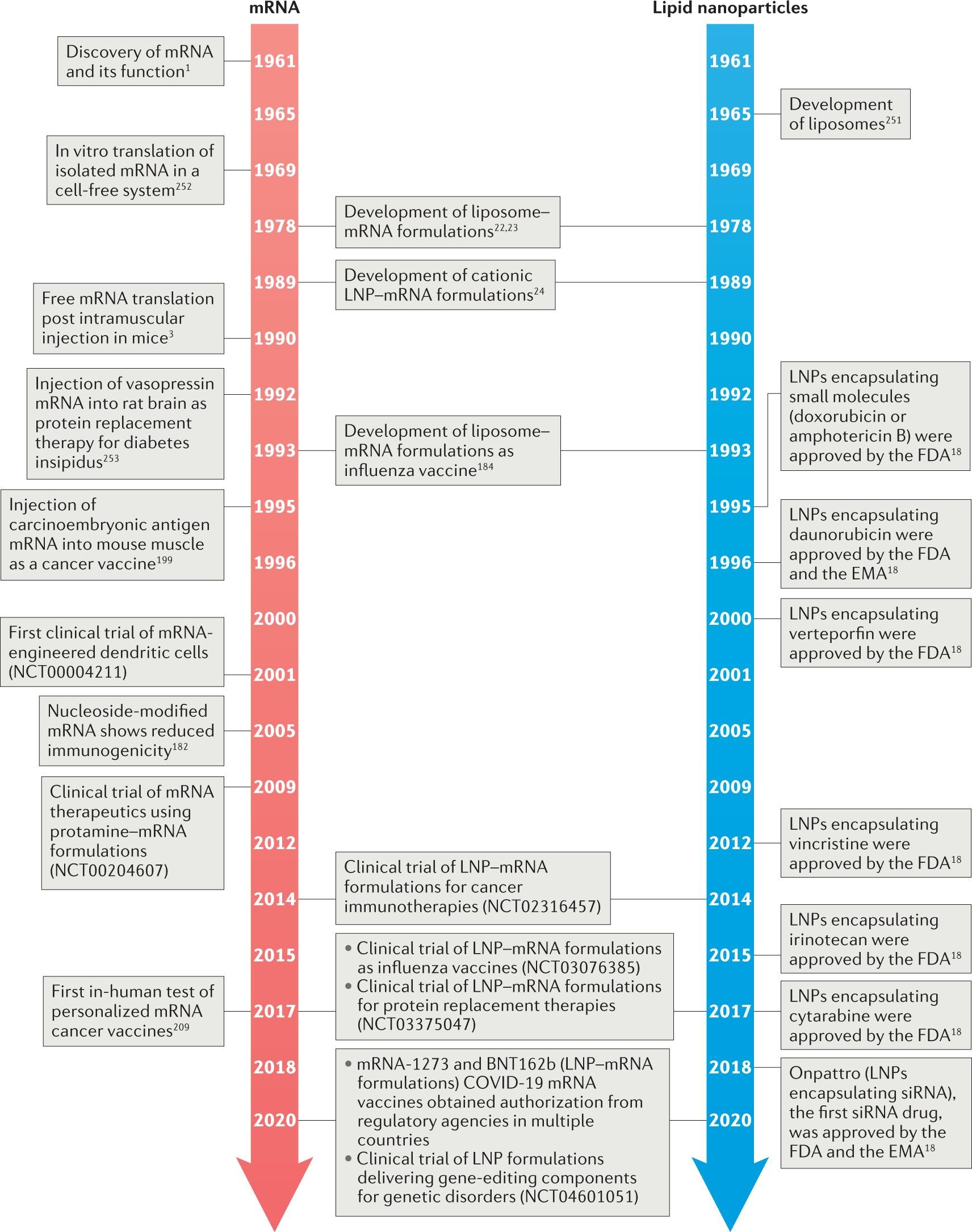
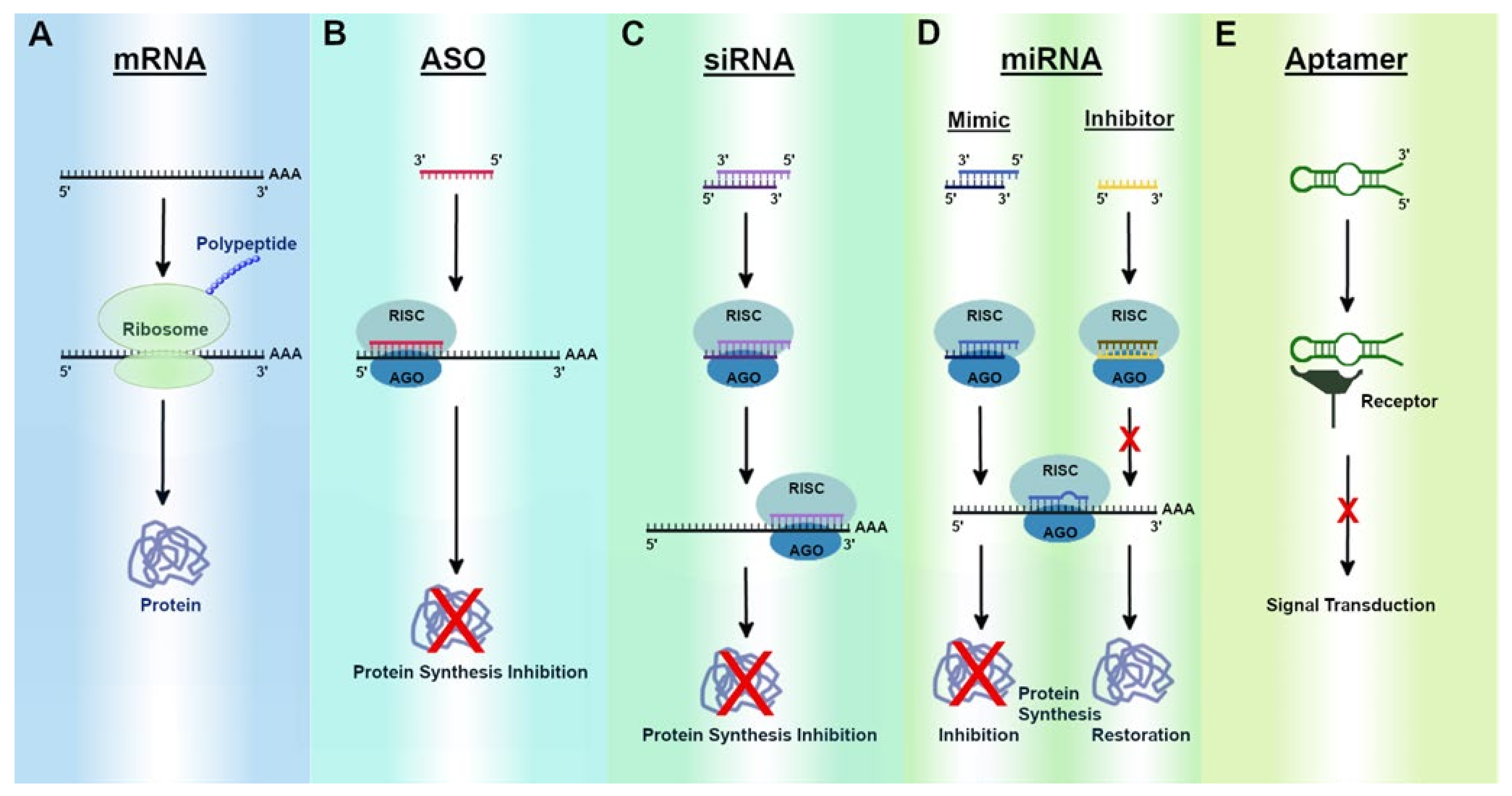
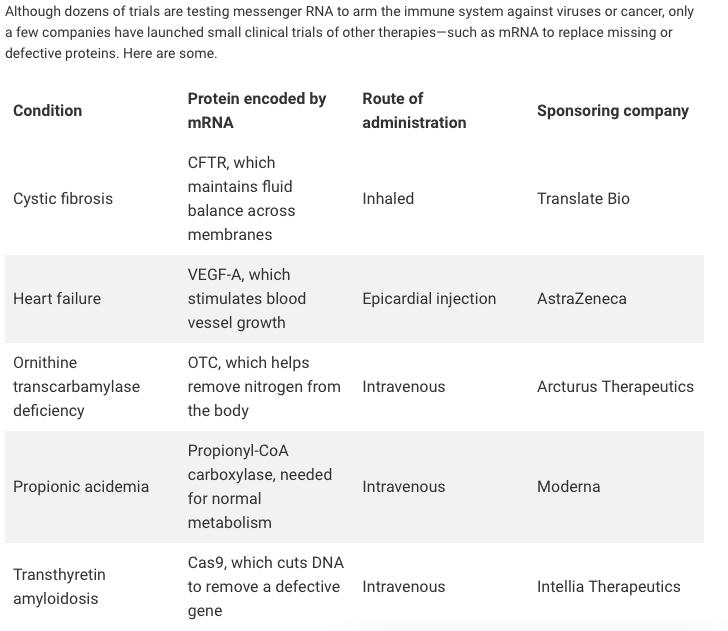
Recent Developments in mRNA-Based Protein Supplementation Therapy to Target Lung Diseases: Molecular Therapy

Kate Eveling on Twitter: "🫁 BiomX are developing BX004 Phage therapy, viruses that target & kill specific bacteria, in this case chronic Pseudomonas aeruginosa. They are advancing into Phase 1b/2a trial 🫁Beyond

GeneFo - A Social Medical Platform - The FDA has granted the go-ahead to begin a clinical trial for MRT5005 which is a revolutionary CF therapy delivering mRNA encoding with fully functional
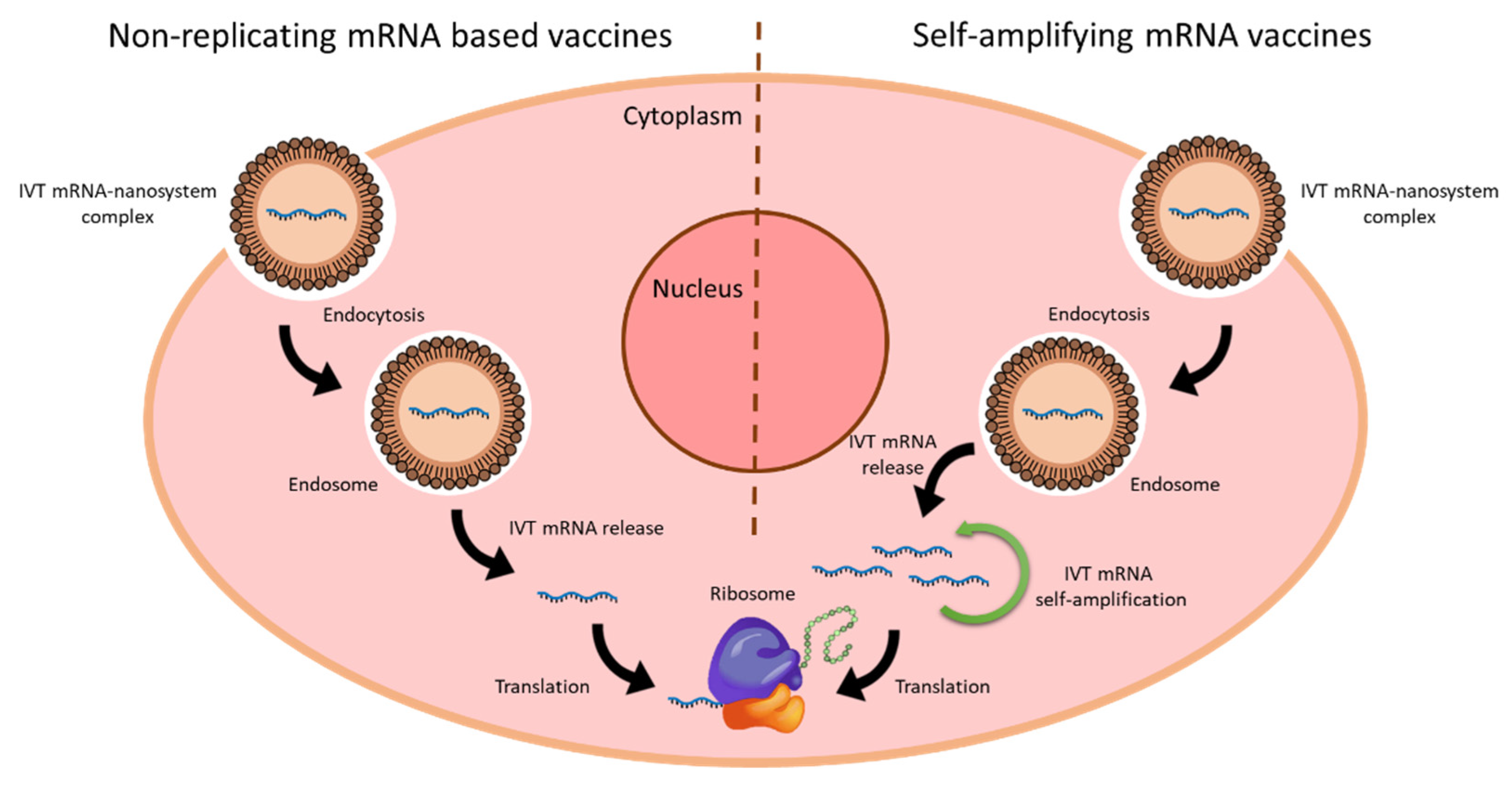
Nanomaterials | Free Full-Text | Nanomedicines to Deliver mRNA: State of the Art and Future Perspectives | HTML
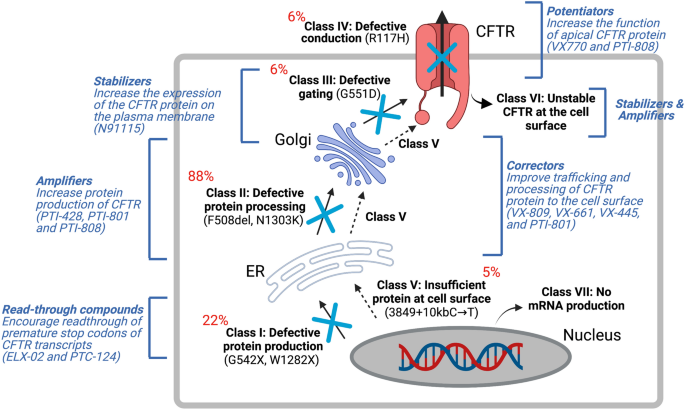
Gene therapy for cystic fibrosis: new tools for precision medicine | Journal of Translational Medicine | Full Text
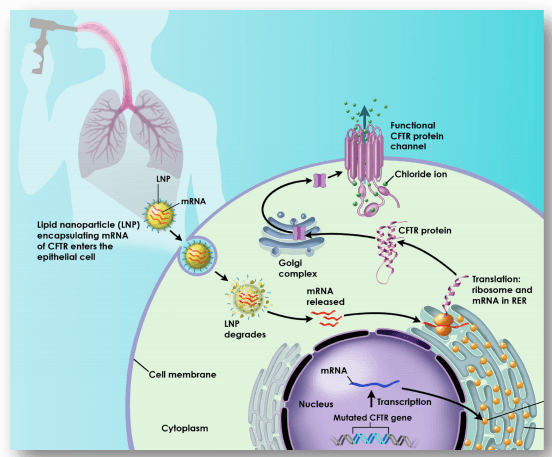
Translate Bio: New Management, Aggressive mRNA Pipeline, And Substantial Upside (TBIO) | Seeking Alpha

FDA Grants Fast Track Designation for Translate Bio's MRT5005 for the Treatment of Cystic Fibrosis | Specialty Pharma Journal
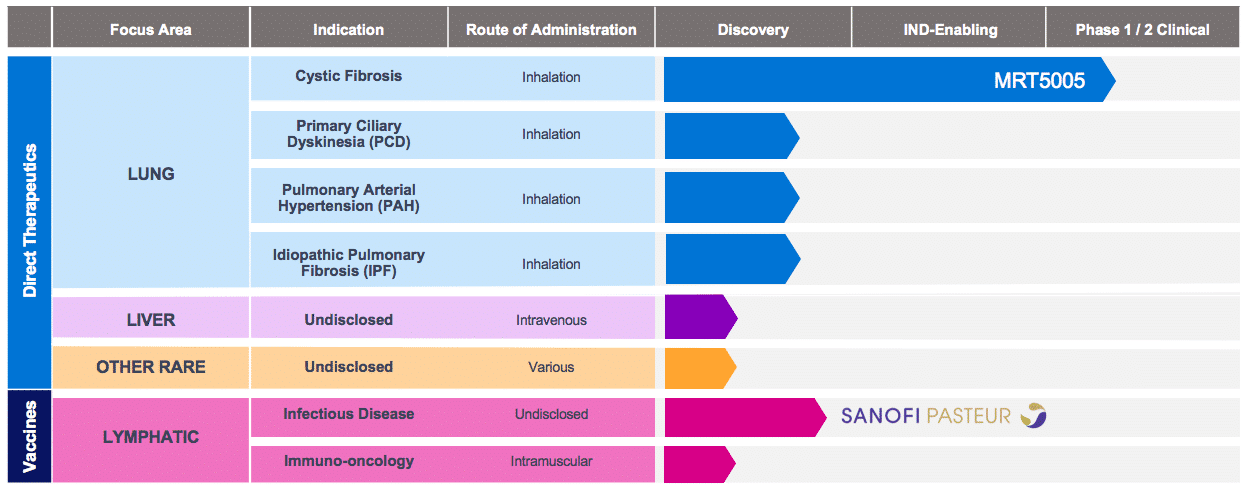
Translate Bio Should Be On Your Radar With Upcoming Presentation And CF Data In 2020 (NASDAQ:SNY) | Seeking Alpha

![Top 10 Cystic Fibrosis Clinical Trials [2022 Studies] | Power Top 10 Cystic Fibrosis Clinical Trials [2022 Studies] | Power](https://pwr-og-img.withpower.com/Cystic%20Fibrosis%20research%20studies%20recruiting%20patients%20in%202022%20need%20your%20help.%20Receive%20premium%20care%20&%20cutting%20edge%20treatments%20by%20enrolling%20in%20cystic%20fibrosis%20clinical%20trials%20today..png?md=1)