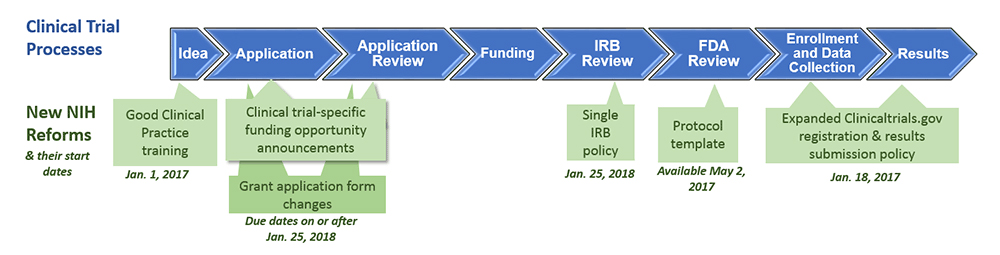
Atlanta Pediatric Research | NIH Requirements for Human Subject Research | Clinical Research Resources | Research Resources | Research | Emory + Children's + GT | Atlanta Pediatric Research Alliance

CTA — Clinical Research Blog | Certified Clinical Research Professionals Society - Clinical Research Certification

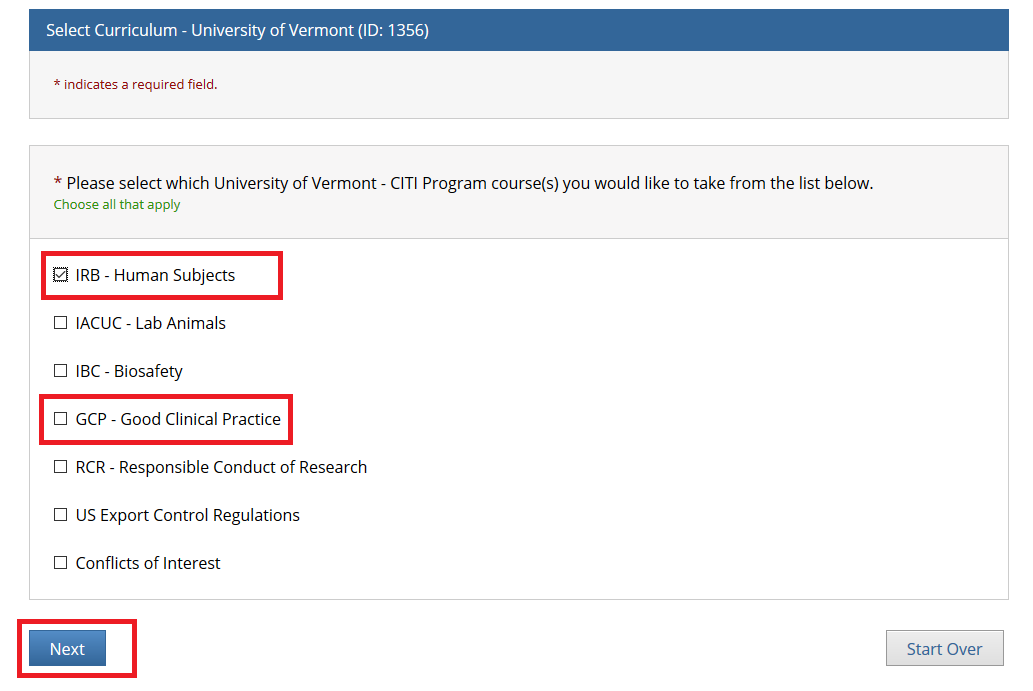
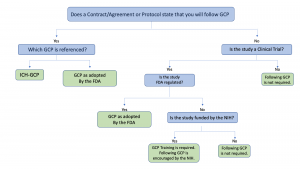
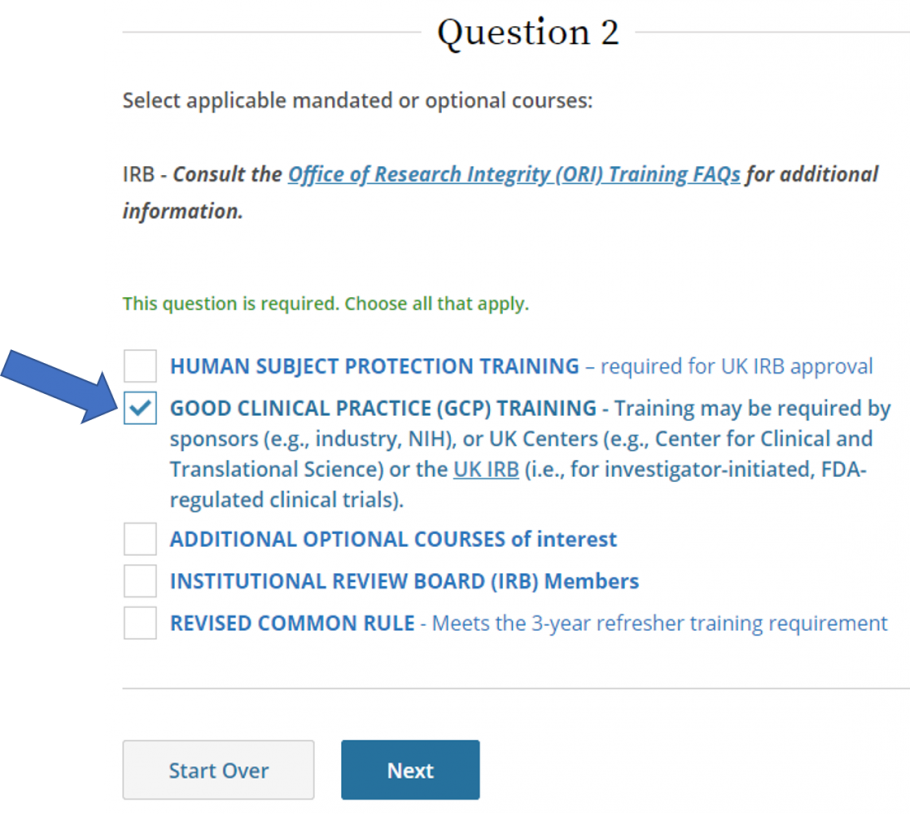
Instructions for Completing Good Clinical Practices Training - Office of Research Support and Compliance
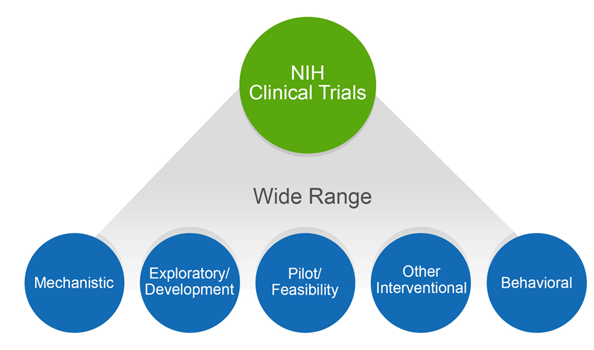
4 Questions For Researchers and Institutions Involved In Human Subjects Research – NIH Extramural Nexus