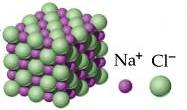
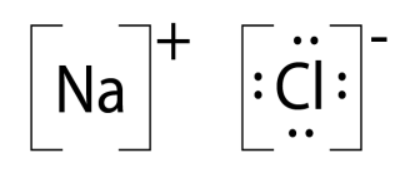Sodium Chloride Is An Ionic Compound With The Chemical Formula NaCl, Representing A 1 To 1 Ratio Of Sodium And Chloride Ions. 3d Illustration Stock Photo, Picture And Royalty Free Image. Image 94737086.

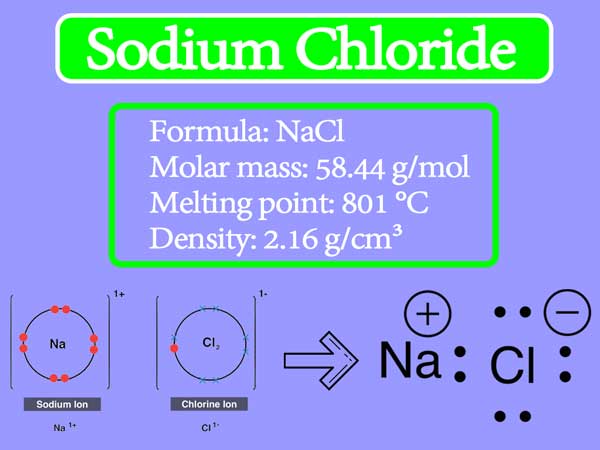
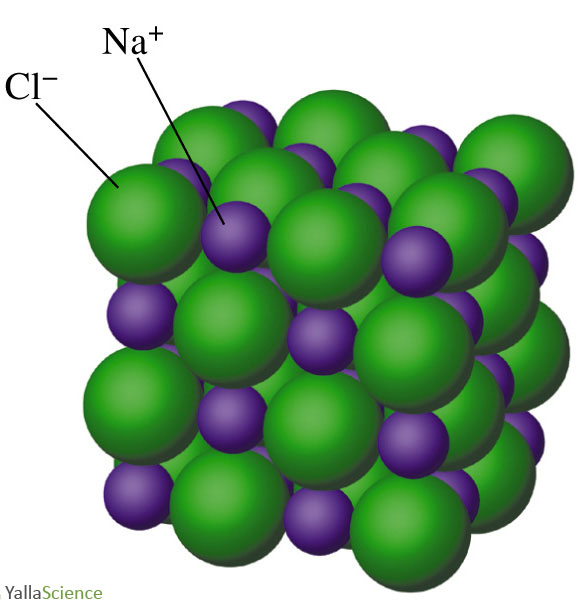
Molecular Structure of Sodium Chloride on White Background Stock Illustration - Illustration of mineral, salt: 109065339

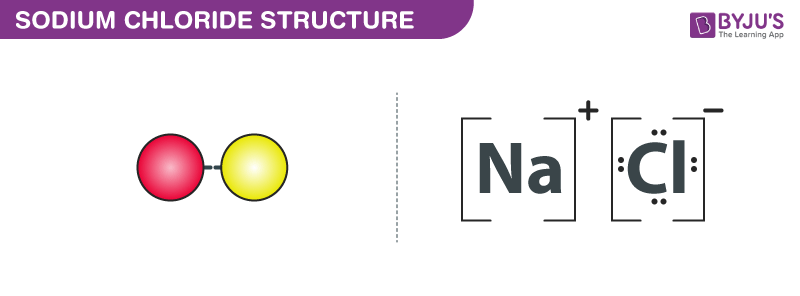
Lewis structure of NaCl - How to draw Lewis structure of NaCl, Polar or Non-polar molecule, and Crystal Structure with FAQs

Sodium Chloride Is An Ionic Compound With The Chemical Formula NaCl, Representing A 1 To 1 Ratio Of Sodium And Chloride Ions. 3d Illustration Stock Photo, Picture And Royalty Free Image. Image 94761907.

Sodium Chloride Nacl Molecule Simple Molecular Stock Vector (Royalty Free) 1904252893 | Shutterstock
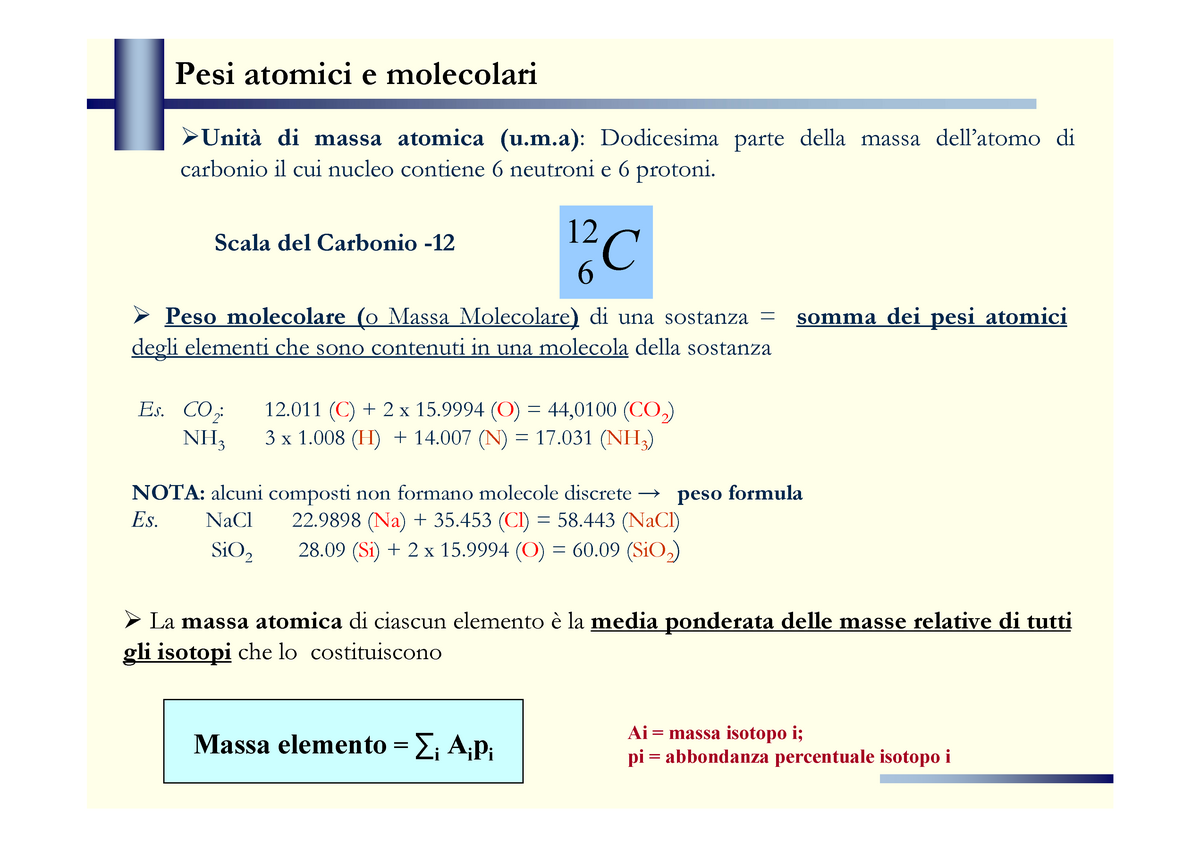
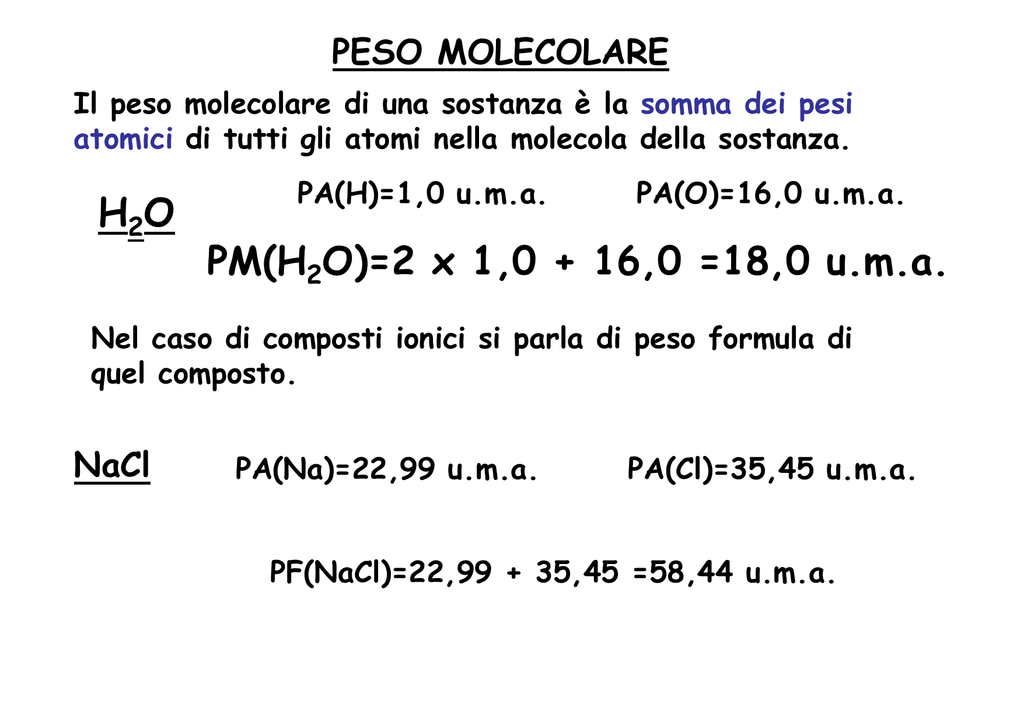
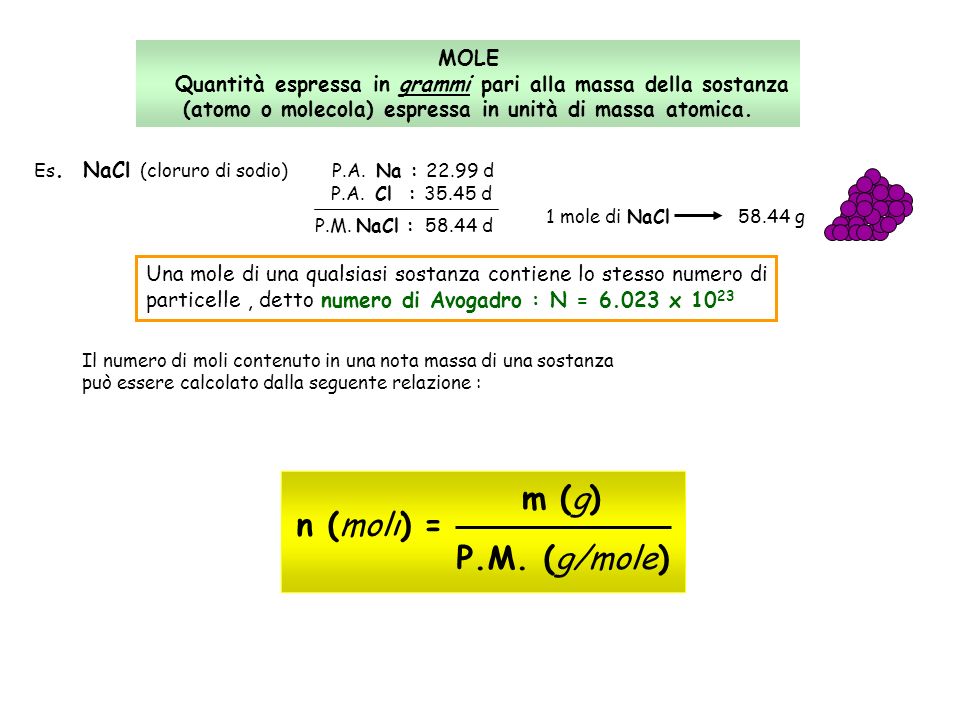
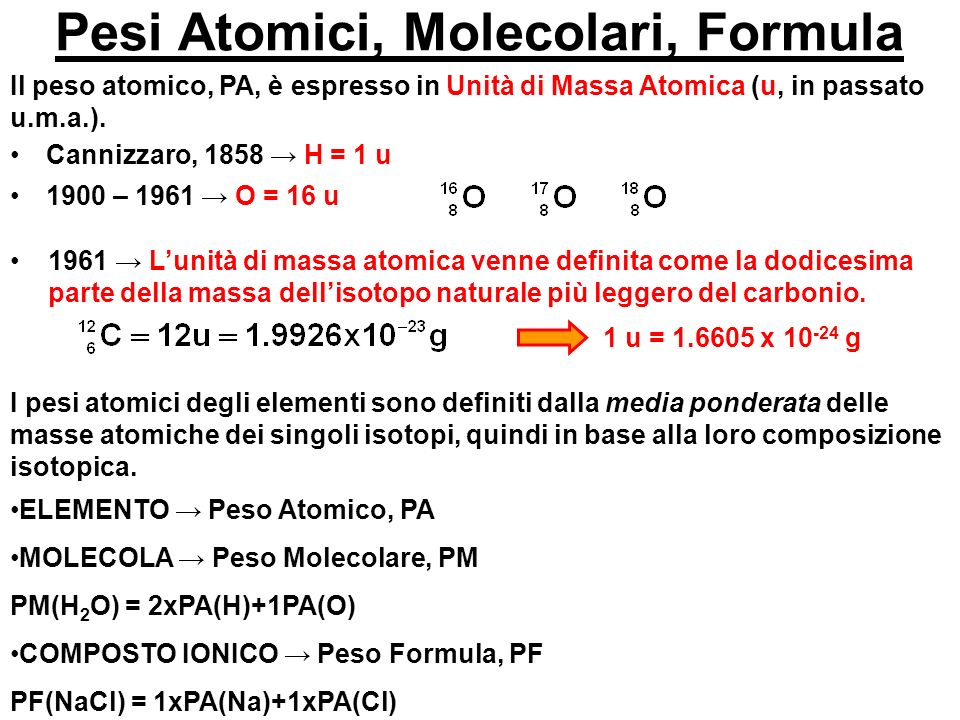
Es1 Stechiometria - Pesi atomici e molecolari di massa atomica (u.m): Dodicesima parte della massa - StuDocu

MASSE ATOMICHE Difficoltà legate alla conoscenza della formula molecolare. Se l'acqua fosse HO avremmo la massa dell'ossigeno pari a 7,9367 g. Inizialmente. - ppt scaricare
Molecular Structure of Sodium Chloride on White Background Stock Illustration - Illustration of crystal, molecule: 109065341